“You may choose to look the other way, but you can never say again that you did not know.”— William Wilberforce
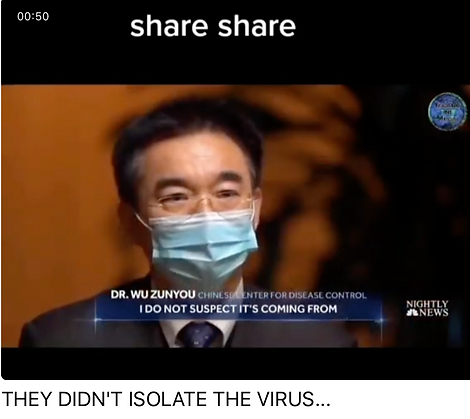
Italy revises its figures for convid deaths from 130,000 to less than 4000


Another one of his emails
Masks are for the masses, not the 'elite'.

Click the picture to watch the video
"As a vaccine developer, I am not fully confident about what's happened over the last 18 months. I can't tell you in 10 years if there will be a side effect of a mRNA vaccine or there won't be."
Professor Petrovsky's Career:
Flinders University South Australia
• College of Medicine and Public Health
• Director of Endocrinology – T2D and Vaccine research
• Professor of Medicine
• Founder of a vaccine development company funded by USA National Institute of Health (NIH) to develop novel vaccine technologies.
• 1979 - Bachelor of Medical Science
• 1982- Bachelor of Medicine
• 1998 - PhD
Has been involved in the development of vaccines for:
1. Influenza
2. Hepatitis B
3. Sting Allergy
4. Malaria
5. Japanese Encephalitis
6. Rabies
7. HIV
Authored 90 scientific papers and book chapters.
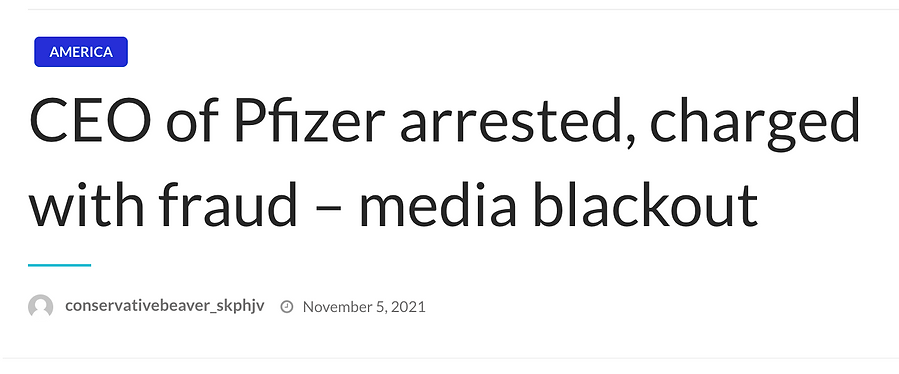
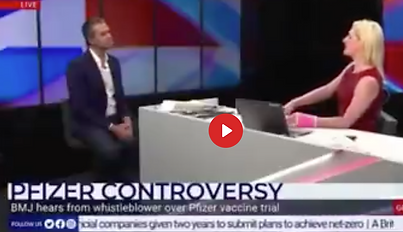
Research Director, who shares concerns over 'poor practices' during Pfizer trials, is sacked
"Revelations of poor practices at a contract research company helping to carry out Pfizer’s pivotal covid-19 vaccine trial raise questions about data integrity and regulatory oversight. Paul D Thacker reports"
"researchers who were testing Pfizer’s vaccine at several sites in Texas during that autumn, speed may have come at the cost of data integrity and patient safety. A regional director who was employed at the research organisation Ventavia Research Group has told The BMJ that the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer’s pivotal phase III trial. Staff who conducted quality control checks were overwhelmed by the volume of problems they were finding. After repeatedly notifying Ventavia of these problems, the regional director, Brook Jackson, emailed a complaint to the US Food and Drug Administration (FDA). Ventavia fired her later the same day. Jackson has provided The BMJ with dozens of internal company documents, photos, audio recordings, and emails."
More from the BMJ
"Concerns raised
In her 25 September email to the FDA Jackson wrote that Ventavia had enrolled more than 1000 participants at three sites. The full trial (registered under NCT04368728) enrolled around 44 000 participants across 153 sites that included numerous commercial companies and academic centres. She then listed a dozen concerns she had witnessed, including:
-
Participants placed in a hallway after injection and not being monitored by clinical staff
-
Lack of timely follow-up of patients who experienced adverse events
-
Protocol deviations not being reported
-
Vaccines not being stored at proper temperatures
-
Mislabelled laboratory specimens, and
-
Targeting of Ventavia staff for reporting these types of problems."